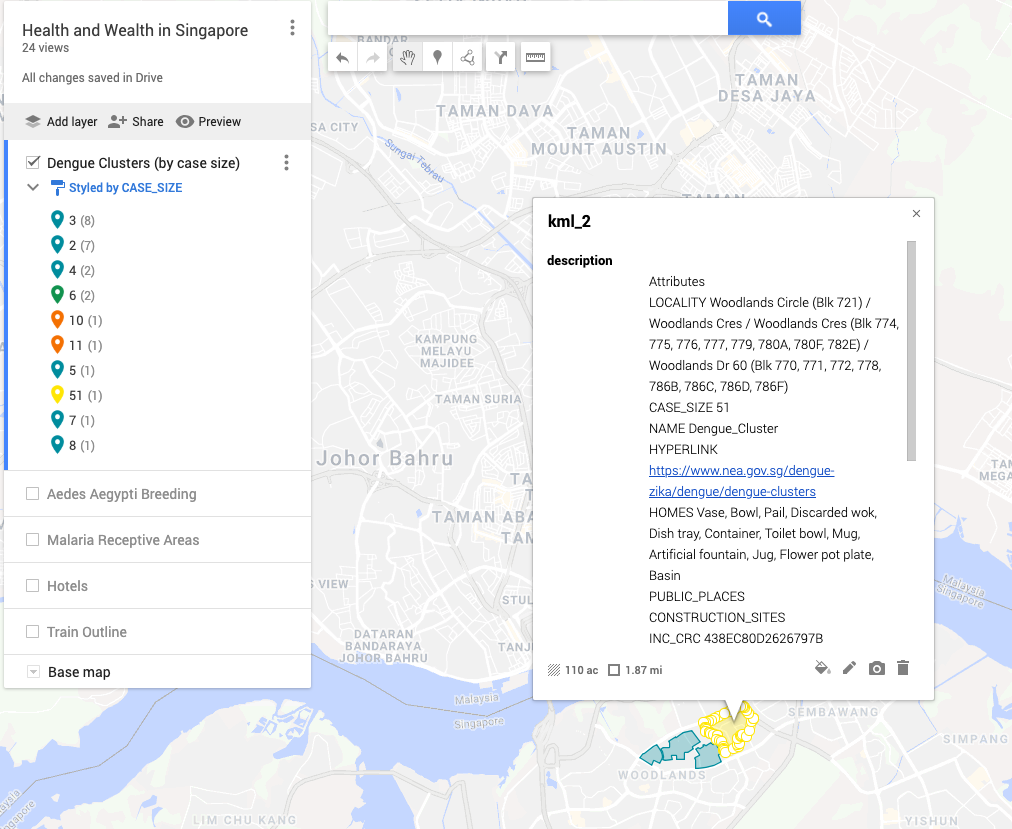
If you have even the foggiest memory of high school biology, you most likely, at some point, were acquainted with Gregor Mandel. He was an Austrian monk and naturalist who had a particular fondness for peas. Ring any bells? If not here’s a refresher: he found that when he crossed a purebred yellow pea plant with a purebred green pea plant, the offspring were all yellow. He then let those offspring self-fertilize and found that for every three yellow plants, there was one yellow one. From this little experiment, he deduced that some traits, like the “yellow” plant color in peas, are dominant over other traits like the “green” plant color. Moreover, he identified that each parent provided a factor (we now call them genes and call the set of their variants, alleles) that determined how the trait would appear in offspring, and that these factors are passed along independently of one another. Here’s a diagram to help make it more clear:
Got it? Good. Well, I’m here to tell you that scientists have been working on technologies that tease and prod the rules of Mendelian inheritance and they’re doing it with a kind of technology called a gene drive. Gene drives bias the inheritance of certain alleles so that those alleles are driven throughout a population.
The idea of building a way to drive a specific gene throughout an entire population is not new, selfish genes, or genes that bias their own transmission, are found throughout nature.
In the 1960s, scientists imagined how we might take advantage of genetics to confer immunity to pathogens in insect pests. The idea of using genetics to manage wild populations gained more interest after Austin Burt in 2003 described the kind tool necessary to make it happen; a kind of “find and replace” for DNA.
In 2012, researchers finally had that tool — CRISPR. CRISPR, and its family of associated proteins (Cas), represent a big step up in how scientists find, cut, and replace genomic DNA. Its easier and more efficient to use than other techniques and is cheaper to boot. CRISPR is found in nature as a way for bacteria to defend themselves from viruses by maintaining short segments of viral DNA, as a sort of memory of infection, to use as a cutting pattern. Scientists can use this system to target and cut virtually any piece of DNA which can then be repaired to include virtually any other piece of DNA.
It’s important not to understate how remarkable it is to engineer a particular trait to be passed along hereditarily and this is true for two critical reasons. The first is that whenever we attempt to alter the genetics of an organism in the wild, the result is almost guaranteed to be less fit than its unaltered counterparts. This is because, fitness, or the ability to successfully compete and reproduce in the wild, is the lever by which natural selection works. The many forms of life we see around us are products of evolution, optimized for a given environment, and thus the winners of a competition that’s been happening since the dawn of life. The second is the biological process that passes on genes from each parent to the offspring happens on an incredibly short time scale. The entire process of finding, cutting and replacing genomic DNA has to be “turned on” at exactly the right time. Too early, and you might cause unintended effects that hurt fitness more, too late, and the window passes for the editing to be effectively inherited.
After reading that you might be wondering: how practical and/or feasible is it that gene drives will get made and released? Well, Gene drives could be very effective in slowing or eliminating the potential of vector-borne diseases spread by insects like Lyme, dengue, Zika, or malaria. As malaria alone is responsible for over 400,000 deaths a year and the mosquitos that are responsible serve no unique niche, there’s a strong incentive to develop the technology. In laboratory settings, scientists have been able to use a gene drive to crash a population of the malaria vector. For species with long reproductive cycles, like humans, it would take an impractical amount of time, on the order of centuries or millennia. In agriculture, it may be useful to create localized drives to suppress the populations of pests like weeds. That being said, in the case of domesticated livestock, much of the reproductive cycle is already closely overseen by traditional methods and commercial-scale livestock already suffer greatly from a fitness disadvantage because of it.
Genome editing is still in its infancy — there is still much to discovered and discussed about how and when it used. Time will tell if gene drives will live up to the attention they have received.